What is a bioferment?
A bioferment, or a product generated by biofermentation, bioconversion, or other bioprocessing techniques, is derived from the metabolic activity of living microorganisms. These microbes “manufacture” new compounds at the cellular level, with over 50 billion of these “biological factories” per liter in the bioreactor.
Some common microbes utilized for the biosynthesis of compounds for skin and hair applications include yeast (Saccharomyces) and bacteria (Lactobacillus and Bifidobacterium).2 These microorganisms are put to work, processing biomass and carbon sources (i.e. sugar) to yield the desired active ingredients.
The compounds produced in a bioferment are controlled by the carbon source (what the organisms are fed) and other key conditions. Biofermentation and bioconversion processes can utilize many feedstocks, including simple sugars or more complex carbon sources such as biomass (plants and algae). For example, aloe, rice, soybeans, blackberries, and wasabi roots have been explored for cosmetic application.1 Seaweeds, including red, brown, and green marine algal species, are also suitable candidates for bioconversion by microbial digest.2
In addition to the carbon source, the microbes’ growth conditions profoundly impact the resulting bioferment. Managing these conditions (such as temperature, light, agitation, atmosphere, and pH) influences specific metabolic pathways to generate particular compounds. For example, bioreactor temperature modulation can induce gene expression in the microorganisms, producing protective, restorative molecules that support cellular recovery. Each specific biofermentation protocol is developed to achieve a balance of the desired bioactive ingredients.
Continuous growth of bioferments
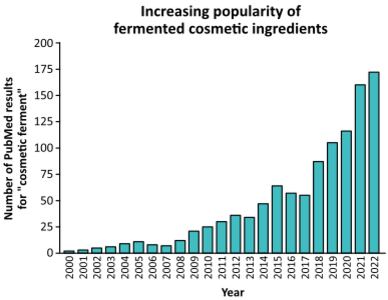
According to reports, fermented ingredients are trending, especially in the personal care industry. Projections suggest bioferments will expand to a $93.9 billion market globally in the next decade with an impressive compound annual growth rate (CAGR) of 6.7%.3 At the same time, researchers are actively exploring the possibilities of biofermentation. As illustrated below, the number of publications on biofermented cosmetic ingredients continues to grow annually.
The microbes behind biofermentation
Saccharomyces cerevisiae is a popular microbe in biofermentation methodologies. Commonly known as baker’s or brewer’s yeast, S. cerevisiae is critical for producing bread, beer, wine, and many other favorite foods. In addition, this yeast is frequently used for biomanufacturing compounds for the personal care industry.
Yeast is a fungus, evolutionarily distinct from plants and animals. There are over 1,500 different yeast species (including genera such as Saccharomyces and Candida) that comprise only about 1% of all fungal species.4
Fun fact: Yeasts do not form mushrooms to reproduce, as some multi-cellular fungi do. Instead, single-celled microscopic yeasts propagate by splitting (either budding or fission).
One thing yeast and mushroom-forming fungi do have in common is how they obtain nourishment. While animals consume food and plants photosynthesize, fungi can secrete enzymes to digest nearby food sources before absorbing the released nutrients.
Big benefits with biofermentation
The value proposition and return on investment of bioferments come from many factors, including sustainability, reproducibility, scalability, and low carbon footprint compared to traditional chemical synthesis and other synthetic processes.
High biofunctionality: Ingredients made through biofermentation processes can support sought-after beauty claims in skin care and hair (scalp) care, such as antioxidant, anti-photoaging, anti-wrinkle, anti-inflammatory, skin brightening, and moisturization effects.2
Enhanced bioavailability: Biofermentation often enhances the bioavailability of actives with improved absorption, compatibility, and activity compared to non-fermented products. For example, large macromolecules cannot pass through the skin barrier, but microorganisms readily break these down into smaller, more absorbable compounds. Biofermentation can also increase bioavailability and biocompatibility by optimizing the molecular weight, solubility, pH, polarity, lipophilicity, and potency of actives.5
Fortified with nutrients: Microorganisms “digesting” biomass liberate peptides, enzymes, antioxidants, vitamins, and other valuable nutrients.
Flexibility & accessibility: A single bioreactor can be used to produce many products, each using unique microbes and carbon sources, instead of a purpose-built, large-footprint single-product facility. Additionally, the tools and techniques needed for biofermentation have become more readily available and affordable.
Greater sustainability: Bioferments are often more sustainable with lower environmental impact. Producing actives through biofermentation provides opportunities for upcycling waste for circular beauty, leaving a smaller carbon footprint.2
Novel, innovative ingredients: Biofermentation can avoid the need for chemical synthesis or organic extraction by relying on “biological factories” instead. In some cases, biofermentation methods can be superior for concentrating desired compounds.
Unlocking the photoprotective powers of Sargassum with yeast biofermentation
Sun-loving seaweeds protected by phlorotannins
Sargassum vulgare thrives in the Sargasso Sea. Mats of this brown algae float at the water’s surface, soaking in the energy from the intense sunlight. While the relentless rays nourish the growing algal masses, the sun also delivers harsh ultraviolet (UV) radiation. This high-energy UV light damages DNA, which can induce mutagenic and cytotoxic DNA lesions, and increases cell oxidative stress.

To survive in their sunny environment, Sargassum vulgare has evolved powerful, UV-protectant molecules known as phlorotannins (polymers of phloroglucinol; 1,3,5-trihydroxy-benzene).6 A mixture of soluble and insoluble phlorotannins is found throughout Sargassum’s leafy appendages. Research has demonstrated that these phenolic compounds absorb UV to mitigate the DNA damage brought on by the incessant UV exposure experienced at the sea’s surface.7-9 In fact, algae produce more phlorotannins in response to UV exposure, which facilitates a reduction of UV-induced DNA damage, including cyclobutane pyrimidine dimers (CPDs) and pyrimidine 6–4 pyrimidone photoproducts (6-4PPs).7
From Sargassum to skincare
Biocogent sought to harness Sargassum’s natural photoprotective activity. Unfortunately, classic solvent-based extraction methods cannot liberate functional bioactives and are least preferred. Therefore, biofermentation is an ideal method for sustainably producing DermalRx FSE.
A novel bioconversion of Sargassum vulgare by a proprietary strain of Saccharomyces cerevisiae yeast delivers a potent product that protects and repairs sun-exposed skin. DermalRx FSE is rich in beneficial compounds, including bioavailable phlorotannins, with optimal stability.
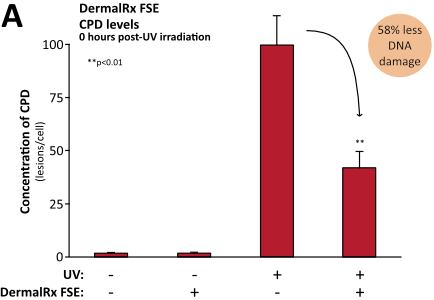
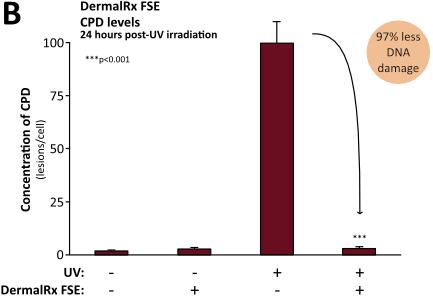
Numerous studies demonstrate DermalRx FSE’s ability to mitigate DNA damage in human skin after exposure to UV radiation, as evidenced by reduced CPD levels.10 Immediately following UV exposure, DermalRx FSE-treated tissues had 58% less CPD than untreated controls (Figure, panel A). In addition to preventing DNA damage, DermalRx FSE was also found to stimulate the repair of UV-damaged DNA. Remarkably, in 24 hours, CPD levels dropped 97% with DermalRx FSE compared to untreated samples (Figure, panel B). The ability of DermalRx FSE to reduce CPD was also visualized in sections of a 3D skin model.10
Notably, further analysis revealed that DermalRx FSE regulates four microRNAs to elevate DNA repair pathways, including nucleotide excision repair (NER), base excision repair (BER), and double-strand break (DSB) repair.10 Along with delivering photoprotective effects, DermalRx FSE also provides strong antioxidant and inflammatory protection.10
DermalRx FSE exemplifies what is possible when using biofermentation technologies to develop and manufacture potent, biofunctional materials.
To learn more about DermalRx FSE from the biofermentation specialists at Biocogent, contact info@biocogent.com.
References
1 Sivamaruthi, B. S., Kesika, P., Prasanth, M. I. & Chaiyasut, C. A Mini Review on Antidiabetic Properties of Fermented Foods. Nutrients 10 (2018). https://doi.org:10.3390/nu10121973
2 Majchrzak, W., Motyl, I. & Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 27 (2022). https://doi.org:10.3390/molecules27154845
3 Fermented Ingredients Market,
4 Kurtzman, C. P. & Piškur, J. in Comparative Genomics: Using Fungi as Models (eds Per Sunnerhagen & Jure Piskur) 29-46 (Springer Berlin Heidelberg, 2006).
5 Kim, B. et al. Transdermal delivery systems in cosmetics. Biomedical Dermatology 4, 10 (2020). https://doi.org:10.1186/s41702-020-0058-7
6 Lomartire, S. & Gonçalves, A. M. M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar Drugs 20 (2022). https://doi.org:10.3390/md20020141
7 Gomez, I. & Huovinen, P. Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochem Photobiol 86, 1056-1063 (2010). https://doi.org:10.1111/j.1751-1097.2010.00786.x
8 Prasedya, E. S. et al. UVA Photoprotective Activity of Brown Macroalgae Sargassum cristafolium. Biomedicines 7 (2019). https://doi.org:10.3390/biomedicines7040077
9 Birkemeyer, C., Lemesheva, V., Billig, S. & Tarakhovskaya, E. Composition of Intracellular and Cell Wall-Bound Phlorotannin Fractions in Fucoid Algae Indicates Specific Functions of These Metabolites Dependent on the Chemical Structure. Metabolites 10 (2020). https://doi.org:10.3390/metabo10090369
10 Scacchi, B., Costello, B., Ceccoli, J. & Lawrence, P. Brown Seaweed Ferment Propagates UV Damage Protection for Skin. Cosmetics & Toiletries 137, DM19-DM29 (2022).


